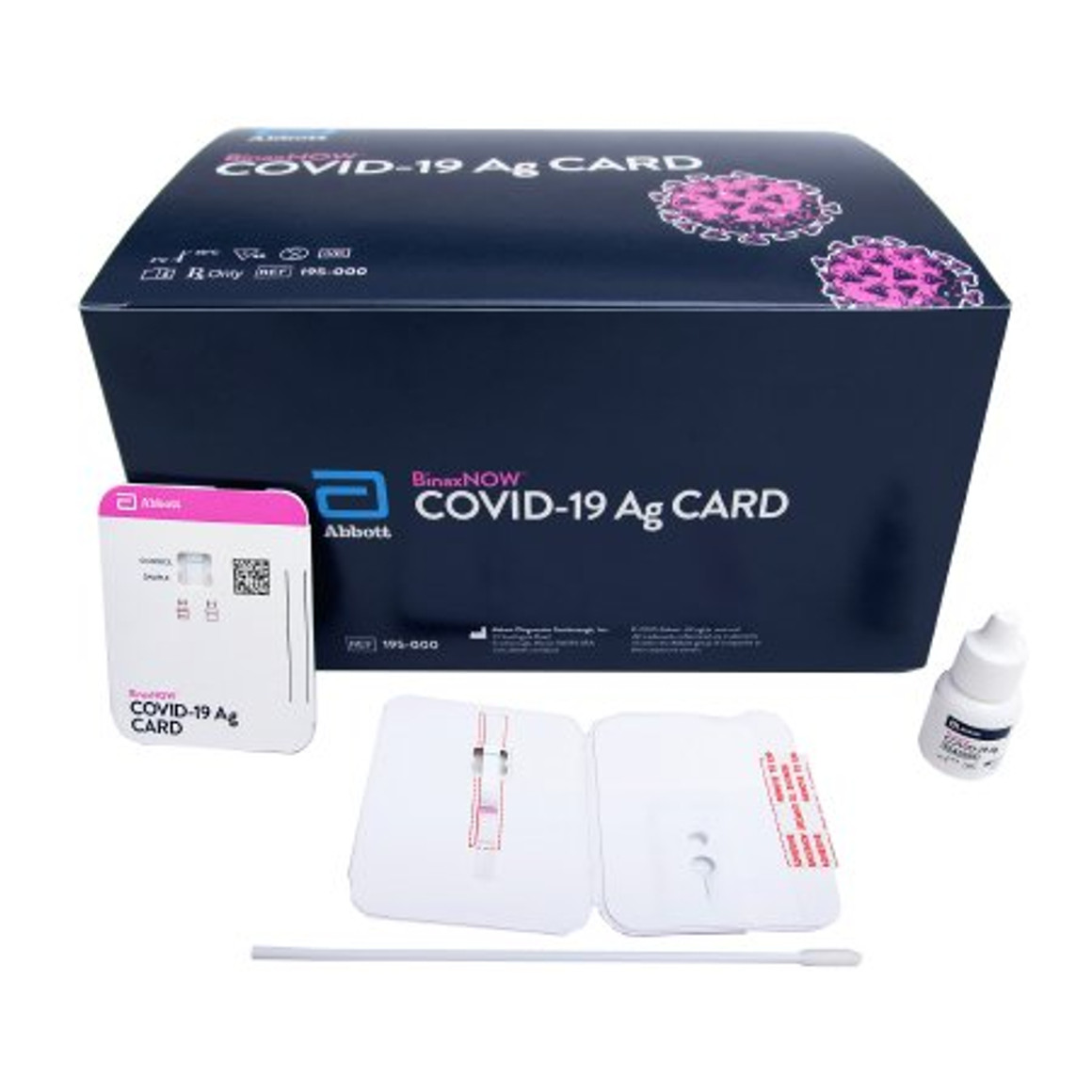
BinaxNOW™ Professional Use Antigen Detection COVID-19 Ag Nasal Swab Sample 40/Pk
Was:
$832.95
Your Price:
$336.95
- MFR:
- 195000
- Medex SKU:
- ADE-195000
- Packing Info:
- 40/Pack
- Usually Ships:
- 4 - 6 Weeks
- Freight Quote Button:
- False
- Notice:
- Due to regulatory requirements, this item can only be shipped to customers who have a valid Medical license on file. To add your license information, please click here
Description
- BinaxNOW COVID-19 Ag Card is only for use under the Food and Drug Administration’s Emergency Use Authorization: https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations
- Testing is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high or waived complexity tests; this test is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation
- The BinaxNOW COVID-19 Ag Card is a lateral flow immunoassay for the qualitative detection of the nucleocapsid protein antigen to SARS-CoV-2 directly from anterior nasal (nares) swab specimens collected from individuals who are suspected of COVID-19 by their healthcare provider within seven days of the onset of symptoms
- Laboratories within the United States and its territories are required to report all results to the appropriate public health authorities
- Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status
- Positive results do not rule out bacterial infection or co-infection with other viruses
- Negative results should be treated as presumptive and confirmation with a molecular assay, if necessary, for patient management, may be performed
- Negative results do not rule out SARS-CoV-2 infection and should not be used as the sole basis for treatment or patient management decisions, including infection control decisions
- The BinaxNOW COVID-19 Ag Card is intended for use by medical professionals or trained operators who are proficient in performing rapid lateral flow tests
- The BinaxNOW COVID-19 Ag Card does not differentiate between SARSCoV and SARS-CoV-2
- Sensitivity (PPA) 84.6% (entire population)
- Sensitivity (PPA) 95.6% (those with PCR cycle threshold [Ct] < 33)
- Specificity (NPA) 98.5%
- Onboard extraction allows the swab to be directly inserted into the test card
- Visually read results in 15 minutes - no instrument required